The Equilibrium Constants for the Chemical Reaction N2(g)+ O2(g) Hº < 0
B)The Partial Pressure of NO(g)is Less at 2,200 K
The equilibrium constants for the chemical reaction N2(g) + O2(g) 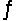
2NO(g) are KP = 1.1 * 10-3 and 3.6 * 10-3 at 2,200 K and 2,500 K, respectively. Which one of these statements is true?
A) The reaction is exothermic, Hº < 0.
B) The partial pressure of NO(g) is less at 2,200 K than at 2,500 K.
C) KP is less than Kc by a factor of (RT) .
D) The total pressure at 2,200 K is the same as at 2,500 K.
E) Higher total pressure shifts the equilibrium to the left.
Correct Answer:
Verified
Q46: The reaction 2SO3(g) Q47: Which of these situations will result if Q48: For the common allotropes of carbon Q49: For the reaction at equilibrium 2SO3 Q50: Consider this reaction at equilibrium at a Q52: Consider the reaction N2(g)+ 3H2(g) Q53: Consider this gas phase equilibrium system: Q54: When the reaction 2H2S(g) Q55: A solution was prepared such that the Q56: Describe why addition of a catalyst does![]()

![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents