Multiple Choice
For the common allotropes of carbon (graphite and diamond) , C(gr) 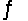 C(dia) with equilibrium constant K = 0.32. The molar volumes of graphite and diamond are, respectively, 5.30 cm3/mol and 3.42 cm3/mol; Hf of diamond is 1.90 kJ/mol. This data suggests that the formation of diamond is favored at
C(dia) with equilibrium constant K = 0.32. The molar volumes of graphite and diamond are, respectively, 5.30 cm3/mol and 3.42 cm3/mol; Hf of diamond is 1.90 kJ/mol. This data suggests that the formation of diamond is favored at
A) low temperatures and low pressures.
B) high temperatures and low pressures.
C) low temperatures and high pressures.
D) high temperatures and high pressures.
Correct Answer:
Verified
Related Questions


