Consider this reaction at equilibrium at a total pressure P1: 2SO2(g) + O2(g) 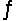
2SO3(g)
Suppose the volume of this system is compressed to one-half its initial volume and then equilibrium is reestablished. The new equilibrium total pressure will be
A) twice P1
B) three times P1
C) 3.5 P1
D) less than twice P1
E) unchanged
Correct Answer:
Verified
Q45: If the reaction 2H2S(g) Q46: The reaction 2SO3(g) Q47: Which of these situations will result if Q48: For the common allotropes of carbon Q49: For the reaction at equilibrium 2SO3 Q51: The equilibrium constants for the chemical Q52: Consider the reaction N2(g)+ 3H2(g) Q53: Consider this gas phase equilibrium system: Q54: When the reaction 2H2S(g) Q55: A solution was prepared such that the Unlock this Answer For Free Now! View this answer and more for free by performing one of the following actions Scan the QR code to install the App and get 2 free unlocks Unlock quizzes for free by uploading documents![]()
![]()

![]()

