Calculate the maximum number of grams of NH3 that can be produced by the reaction of 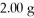
Of N2 with 3.00 g H2.
Reaction: N2(g) + 3 H2(g) → 2 NH3(g)
A) 0.964
B) 2.43
C) 4.00
D) 17.0
E) none of the above
Correct Answer:
Verified
Q83: How many grams of water are theoretically
Q86: How many moles of NH3 can be
Q87: What is the limiting reactant for the
Q88: A 24.0 g sample of nitrogen gas
Q90: What is the limiting reactant for the
Q91: How many moles of aluminum oxide are
Q92: A sample of 8.5 g NH3 on
Q96: What is the limiting reactant for the
Q97: How many grams of the excess reactant
Q98: The reaction for the oxidation of NH3
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents