Which is the limiting reactant in the following reaction given that you start with 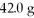
Of CO2 and 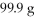
KOH?
Reaction: CO2 + 2KOH → K2CO3 + H2O
A) K2CO3
B) H2O
C) CO2
D) KOH
E) not enough information
Correct Answer:
Verified
Q62: Which ingredient is the limiting reactant if
Q75: If the theoretical yield of a reaction
Q82: What is the excess reactant for the
Q86: How many moles of NH3 can be
Q87: What is the limiting reactant for the
Q88: A 24.0 g sample of nitrogen gas
Q89: If 16.0 grams of aluminum oxide were
Q96: What is the limiting reactant for the
Q98: The reaction for the oxidation of NH3
Q99: How many moles of calcium chloride are
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents