Refer to the figure below. 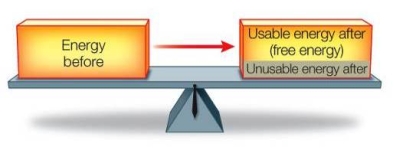 Does this figure illustrate both the first and second laws of thermodynamics?
Does this figure illustrate both the first and second laws of thermodynamics?
A) No, the figure illustrates the first law of thermodynamics only, because it shows how the amount of energy remains constant during a transformation.
B) No, the figure illustrates the second law of thermodynamics only, because it shows how the amount of usable energy decreases as the result of a transformation.
C) No, the figure illustrates the second law of thermodynamics only, because both laws cannot operate simultaneously in the same system.
D) Yes, the figure illustrates both laws of thermodynamics because both laws state the same basic principle.
E) Yes, the figure illustrates both laws of thermodynamics because it shows conservation of total energy and a decrease in usable energy during a transformation.
Correct Answer:
Verified
Q134: Living things use chemical reactions to live,
Q135: The synthesis of the enzyme lipase would
Q136: The ΔG of a reaction is dependent
Q137: Refer to the table below. 
Q138: When potatoes are peeled, the enzyme
Q140: Refer to the figure below. 
Q141: The hydrolysis of sucrose into glucose
Q142: Refer to the table below listing facts
Q143: The energy currency of cells is
A) light.
B)
Q144: Where is the chemical energy that is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents