The diagrams shows a PV diagram for 4.3 g of oxygen gas in a sealed container.The temperature of state 1 is 21°C.What are the temperatures T3 and T4? 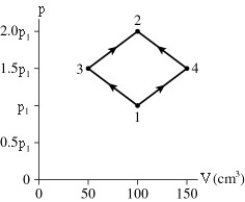
A) -52°C and 390°C
B) 16°C and 47°C
C) 220°C and 660°C
D) 11°C and 32°C
Correct Answer:
Verified
Q20: A hot air balloon has a volume
Q21: 1.0 mol of an elemental solid and
Q22: The figure shows 0.0074 mol of gas
Q23: A 920 g empty iron kettle is
Q24: A 24.0 kg sample of ice is
Q26: 0.20 g of hydrogen gas are held
Q27: Some properties of glass are listed here.
Q28: 148.0 g of water is heated using
Q29: A person consumes a large meal containing
Q30: A 7 kg sample of mercury is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents