What is the theoretical yield of phosphoric acid (H3PO4,98.0 g/mol) when 10.0 g P4O10 (284 g/mol) reacts with excess water (18.02 g/mol) ? P4O10(s) + 6 H2O 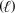 4 H3P4O4(aq)
4 H3P4O4(aq)
A) 10.9 g
B) 40.0 g
C) 10.0 g
D) 13.8 g
E) 2.50 g
Correct Answer:
Verified
Q85: Hemlock contains a number of toxins, one
Q86: A reaction vessel contains equal masses of
Q88: Chemical analysis of a natural organic compound
Q90: You create a process to be used
Q91: You are given a liquid sample that
Q93: How many grams of iron(II,III)oxide (Fe3O4,231.53
Q96: Baking soda (NaHCO3,84.0 g/mol)can react with
Q97: Potassium superoxide (KO2,71.10 g/mol)can be used
Q98: What mass of nitrogen gas (28.02
Q100: What mass of phosphoric acid (H3PO4,98.00 g/mol)is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents