Potassium superoxide (KO2,71.10 g/mol) can be used to generate oxygen gas.What is the theoretical yield of O2 (32.00 g/mol) if 500.0 g KO2 reacts with excess H2O (18.02 g/mol) ? 4 KO2(s) + 2 H2O 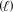 4 KOH(s) + 3 O2(g)
4 KOH(s) + 3 O2(g)
A) 225.0 g
B) 168.8 g
C) 375.0 g
D) 675.1g
E) 56.26 g
Correct Answer:
Verified
Q85: Hemlock contains a number of toxins, one
Q86: A reaction vessel contains equal masses of
Q88: Chemical analysis of a natural organic compound
Q93: How many grams of iron(II,III)oxide (Fe3O4,231.53
Q95: What is the theoretical yield of
Q96: Baking soda (NaHCO3,84.0 g/mol)can react with
Q98: What mass of nitrogen gas (28.02
Q100: What mass of phosphoric acid (H3PO4,98.00 g/mol)is
Q101: Copper metal reacts with nitric acid to
Q102: Mathematically combine the following three reactions
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents