What volume of 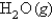 measured at STP is produced by the combustion of 6.27 g of natural gas
measured at STP is produced by the combustion of 6.27 g of natural gas 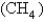 according to the following equation?
according to the following equation? 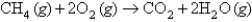
A) 8.76 L
B) 17.5 L
C) 4.38 L
D) 19.1 L
E) 3.14 L
Correct Answer:
Verified
Q69: A 3.31-g sample of lead nitrate,
Q70: What volume of carbon dioxide measured at
Q71: If M is the molar mass,R the
Q72: The standard temperature for gases is
A)100°C
B)0°C
C)32°C
D)212°F
E)0°F
Q73: What volume does 40.5 g of N2
Q75: A mixture is prepared from 15.0 L
Q76: Calculate the density of nitrogen at STP.
A)0.312
Q77: Given the equation: Q78: A mixture of KCl and KClO3 weighing Q79: If a 17.90-g sample of a gas![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents