Given the equation: 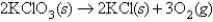 A 3.00-g sample of KClO3 is decomposed and the oxygen at 24.0°C and 0.717 atm is collected.What volume of oxygen gas will be collected assuming 100% yield?
A 3.00-g sample of KClO3 is decomposed and the oxygen at 24.0°C and 0.717 atm is collected.What volume of oxygen gas will be collected assuming 100% yield?
A) 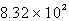 mL
mL
B) 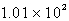 mL
mL
C) 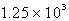 mL
mL
D) 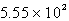 mL
mL
E) none of these
Correct Answer:
Verified
Q72: The standard temperature for gases is
A)100°C
B)0°C
C)32°C
D)212°F
E)0°F
Q73: What volume does 40.5 g of N2
Q74: What volume of Q75: A mixture is prepared from 15.0 L Q76: Calculate the density of nitrogen at STP. Q78: A mixture of KCl and KClO3 weighing Q79: If a 17.90-g sample of a gas Q80: Calcium hydride combines with water according to Q81: Oxygen gas,generated by the reaction Q82: The valve between a 5-L tank containing![]()
A)0.312
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents