Calcium hydride combines with water according to the equation: 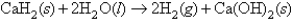 Beginning with 84.0 g of CaH2 and 42.0 g of H2O,what volume of H2 will be produced at 273 K and a pressure of 1327 torr?
Beginning with 84.0 g of CaH2 and 42.0 g of H2O,what volume of H2 will be produced at 273 K and a pressure of 1327 torr?
A) 29.9 L
B) 15.0 L
C) 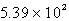 L
L
D) 25.7 L
E) none of these
Correct Answer:
Verified
Q75: A mixture is prepared from 15.0 L
Q76: Calculate the density of nitrogen at STP.
A)0.312
Q77: Given the equation: Q78: A mixture of KCl and KClO3 weighing Q79: If a 17.90-g sample of a gas Q81: Oxygen gas,generated by the reaction Q82: The valve between a 5-L tank containing Q83: What would happen to the average kinetic Q84: You have a 400-mL container containing 55.0% Q85: At 200 K,the molecules or atoms of![]()

Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents