A mixture is prepared from 15.0 L of ammonia and 15.0 L chlorine measured at the same conditions;these compounds react according to the following equation: 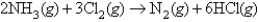 When the reaction is completed,what is the volume of each gas (NH3,Cl2,N2,and HCl,respectively) ? Assume the final volumes are measured under identical conditions.
When the reaction is completed,what is the volume of each gas (NH3,Cl2,N2,and HCl,respectively) ? Assume the final volumes are measured under identical conditions.
A) 0.00 L,5.00 L,7.50 L,45.0 L
B) 5.00 L,0.00 L,5.00 L,30.0 L
C) 0.00 L,0.00 L,7.50 L,45.0 L
D) 0.00 L,0.00 L,5.00 L,30.0 L
E) 0.00 L,10.0 L,15.0 L,90.0 L
Correct Answer:
Verified
Q70: What volume of carbon dioxide measured at
Q71: If M is the molar mass,R the
Q72: The standard temperature for gases is
A)100°C
B)0°C
C)32°C
D)212°F
E)0°F
Q73: What volume does 40.5 g of N2
Q74: What volume of Q76: Calculate the density of nitrogen at STP.![]()
A)0.312
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents