Multiple Choice
A mixture of KCl and KClO3 weighing 1.34 grams was heated;the dry O2 generated occupied 143 mL at STP.What percent of the original mixture was KClO3,which decomposes as follows: 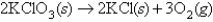
A) 38.9%
B) 58.4%
C) 87.6%
D) 10.7%
E) 23.7%
Correct Answer:
Verified
Related Questions
Q73: What volume does 40.5 g of N2
Q74: What volume of Q75: A mixture is prepared from 15.0 L Q76: Calculate the density of nitrogen at STP.![]()
A)0.312
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents
