The solubility in mol/L of M(OH) 2 in 0.053 M KOH is 1.0 10-5 mol/L.What is the Ksp for M(OH) 2?
A) 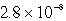
B) 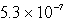
C) 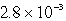
D) 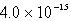
E) 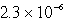
Correct Answer:
Verified
Q54: Given the following Ksp values,which statement about
Q55: The Ksp for BaF2 is 2.4
Q56: A 50.0-mL sample of 0.100 M
Q57: Determine the equilibrium concentration of the
Q58: The following questions refer to the
Q60: The best explanation for the dissolution of
Q61: A solution is 0.010 M in each
Q62: Which of the following solid salts should
Q63: The overall Kf for the complex
Q64: In the qualitative analysis scheme for metal
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents