A reaction is at equilibrium at a given temperature and constant pressure when __________
A) " Srxn" = 0.
B) " S 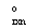 " = 0.
" = 0.
C) " Grxn = 0.
D) " G 11eff76b_8025_8afd_a45e_1396a157067e_TB3833_00" = 0.
E) " Hrxn" = 0.
Correct Answer:
Verified
Q42: Processes are always spontaneous when _
Q44: If a reaction A
Q46: Hydrochloric acid (HCl) reacts with sodium
Q46: The equilibrium vapor pressure for benzene
Q48: The symbol
Q49: At body temperature, many proteins have
Q50: Which of the following statements about
Q60: At constant T and P, any
Q66: The entropy of vaporization of water is
Q75: At what temperature does the Fe(s)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents