If a reaction A B has G 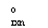 > 0, then when the reaction is at equilibrium there will be __________
> 0, then when the reaction is at equilibrium there will be __________
A) absolutely no B that is formed.
B) smaller quantities of B and larger quantities of A.
C) equal quantities of A and B.
D) large quantities of B and smaller quantities of A.
E) absolutely no A remaining.
Correct Answer:
Verified
Q29: Which of the following is in the
Q39: Indicate which one of the following
Q42: Processes are always spontaneous when _
Q46: Hydrochloric acid (HCl) reacts with sodium
Q47: A reaction is at equilibrium at
Q48: The symbol
Q49: At body temperature, many proteins have
Q60: At constant T and P, any
Q67: A reaction with a low enthalpy
Q75: At what temperature does the Fe(s)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents