The reversible reaction: PCl3(g) + Cl2(g)  PCl5(g) , has K = 0.5. Based on the molecular art shown below, what can be inferred about the reaction conditions?
PCl5(g) , has K = 0.5. Based on the molecular art shown below, what can be inferred about the reaction conditions? 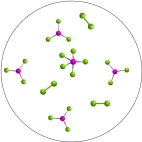
A) The reaction has not yet reached equilibrium.
B) The reaction mixture is at equilibrium.
C) The reaction will shift to the left to reach equilibrium.
D) The forward reaction is favorable.
Correct Answer:
Verified
Q26: Consider the reaction: PCl3(g)+ Cl2(g) 
Q27: Consider the reaction: N2(g)+ O2(g) 
Q28: A catalytic converter uses a catalyst to
Q28: For an endothermic reaction at equilibrium,increasing the
Q29: Which of the following is always necessary
Q30: When the pressure of a reaction at
Q32: What is the gas phase chemical reaction
Q33: Walking at a brisk pace burns off
Q33: The rusting of iron is described
Q36: Which of the following will increase the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents