How many moles of SbCl3 is formed when 4.00 mol Sb are reacted with 4.43 mol Cl2 according to the unbalanced equation 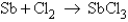
A) 6.65 mol SbCl3
B) 4.43 mol SbCl3
C) 2.95 mol SbCl3
D) 4.00 mol SbCl3
E) Cannot be determined based on the information given.
Correct Answer:
Verified
Q51: Look at the reaction below: 
Q52: For the reaction of C2H4(g) with O2(g)
Q53: Consider the reaction Q54: Determine the mass of CO2 produced when Q55: Consider the reaction Q57: Fe3O4 reacts with CO according to the Q58: Fe3O4 reacts with CO according to the Q59: Consider the equation: Q60: Fe2O3 (molar mass = 159.7 g/mol) reacts Q61: Consider a reaction in which two reactants Unlock this Answer For Free Now! View this answer and more for free by performing one of the following actions Scan the QR code to install the App and get 2 free unlocks Unlock quizzes for free by uploading documents![]()
![]()
![]()

