Look at the reaction below: 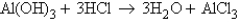 Suppose 0.55 g of water were produced from 1.2 g of aluminum hydroxide and a certain amount of hydrochloric acid. Which of the following statements is true? Choose the best answer.
Suppose 0.55 g of water were produced from 1.2 g of aluminum hydroxide and a certain amount of hydrochloric acid. Which of the following statements is true? Choose the best answer.
A) Aluminum hydroxide is the limiting reactant because 1.2 g of aluminum hydroxide produces 0.55 g of water.
B) Aluminum hydroxide is the limiting reactant because it has a smaller coefficient than the hydrochloric acid.
C) Aluminum hydroxide is not the limiting reactant because more than 0.55 g of water could have been produced from 1.2 g of aluminum hydroxide.
D) Aluminum hydroxide is not the limiting reactant because we are given an amount of leftover aluminum hydroxide.
E) There is not enough information given to answer this question.
Correct Answer:
Verified
Q46: For the reaction of C2H4(g) with O2(g)
Q47: For the reaction Q48: For the reaction Q49: Fe2O3 (molar mass = 159.7 g/mol) reacts Q50: For the reaction Q52: For the reaction of C2H4(g) with O2(g) Q53: Consider the reaction Q54: Determine the mass of CO2 produced when Q55: Consider the reaction Q56: How many moles of SbCl3 is formed Unlock this Answer For Free Now! View this answer and more for free by performing one of the following actions Scan the QR code to install the App and get 2 free unlocks Unlock quizzes for free by uploading documents![]()
![]()
![]()
![]()
![]()

