Determine the mass of CO2 produced when 75.5 g of CaO is reacted with 50.0 g of C according to the unbalanced equation 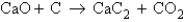
A) 29.6 g CO2
B) 119 g CO2
C) 59.3 g CO2
D) 36.6 g CO2
E) none of these
Correct Answer:
Verified
Q49: Fe2O3 (molar mass = 159.7 g/mol) reacts
Q50: For the reaction Q51: Look at the reaction below: Q52: For the reaction of C2H4(g) with O2(g) Q53: Consider the reaction Q55: Consider the reaction Q56: How many moles of SbCl3 is formed Q57: Fe3O4 reacts with CO according to the Q58: Fe3O4 reacts with CO according to the Q59: Consider the equation: Unlock this Answer For Free Now! View this answer and more for free by performing one of the following actions Scan the QR code to install the App and get 2 free unlocks Unlock quizzes for free by uploading documents![]()

![]()
![]()
![]()

