Passage the N-Terminus of an Amino Acid Must Be Protected with with 9-Fluorenylmethoxycarbonyl
Passage
The N-terminus of an amino acid must be protected with 9-fluorenylmethoxycarbonyl (Fmoc) during solid state peptide synthesis to avoid side reactions (Scheme I) .
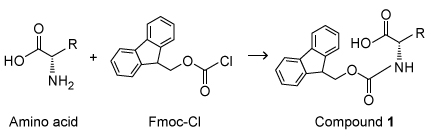 Scheme IThe Fmoc protecting group is typically removed with piperidine (Scheme II) , a heterocyclic secondary amine.
Scheme IThe Fmoc protecting group is typically removed with piperidine (Scheme II) , a heterocyclic secondary amine.
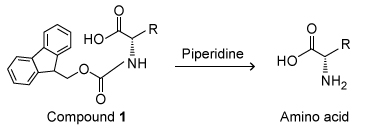 Scheme IIBecause the use of piperidine is restricted, structurally similar amines were examined as possible replacements. To determine whether piperidine analogues were suitable alternatives for Fmoc removal, deprotection of Compound 2 was carried out with several amine bases (Scheme III) .
Scheme IIBecause the use of piperidine is restricted, structurally similar amines were examined as possible replacements. To determine whether piperidine analogues were suitable alternatives for Fmoc removal, deprotection of Compound 2 was carried out with several amine bases (Scheme III) .
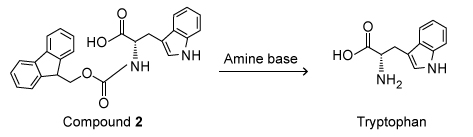 Scheme IIICompound 2 was prepared from L-tryptophan and Fmoc-Cl, and the reaction was monitored by thin-layer chromatography (TLC) and visualized by ultraviolet light. The deprotections were done with piperidine and each analogue using a microwave set to 155 watts at 75 °C for 15 seconds. The products of the deprotections were analyzed by high-performance liquid chromatography (HPLC) and mass spectrometry; the retention times of the products were compared to tryptophan and Compound 2 standards.
Scheme IIICompound 2 was prepared from L-tryptophan and Fmoc-Cl, and the reaction was monitored by thin-layer chromatography (TLC) and visualized by ultraviolet light. The deprotections were done with piperidine and each analogue using a microwave set to 155 watts at 75 °C for 15 seconds. The products of the deprotections were analyzed by high-performance liquid chromatography (HPLC) and mass spectrometry; the retention times of the products were compared to tryptophan and Compound 2 standards.
Adapted from O.P. Luna et al, "Deprotection Reagents in Fmoc Solid Phase Peptide Synthesis: Moving Away from Piperidine?." Molecules. ©2016 MDPI.
-Which of the following statements provides the most plausible explanation for why UV light was used to visualize the thin-layer chromatography plate?
A) The boiling points of the starting materials and products are far enough apart for separation.
B) Tryptophan and Compound 2 will give different molecular ion peaks.
C) The amino acid and Fmoc carbonyl bonds stretch and rotate upon UV light absorption.
D) Conjugated double bonds of the aromatic rings on the indole and Fmoc groups absorb UV light.
Correct Answer:
Verified
Q139: Passage
Solid-phase peptide synthesis is normally carried out
Q140: Passage
Chloramphenicol (Compound 1) is an antibiotic that
Q141: A linear five-carbon ketone has a boiling
Q142: Passage
The protozoan Euglena gracilis expresses glycosyltransferases (enzymes
Q143: Passage
Malaria is caused by the parasite Plasmodium
Q145: Passage
The N-terminus of an amino acid must
Q146: Passage
Malaria is caused by the parasite Plasmodium
Q147: Passage
The N-terminus of an amino acid must
Q148: A carboxylic acid is reacted with NaOH
Q149: Passage
Malaria is caused by the parasite Plasmodium
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents