Passage the N-Terminus of an Amino Acid Must Be Protected with with 9-Fluorenylmethoxycarbonyl
Passage
The N-terminus of an amino acid must be protected with 9-fluorenylmethoxycarbonyl (Fmoc) during solid state peptide synthesis to avoid side reactions (Scheme I) .
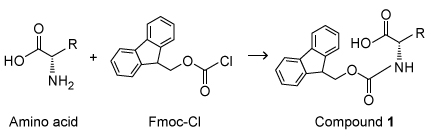 Scheme IThe Fmoc protecting group is typically removed with piperidine (Scheme II) , a heterocyclic secondary amine.
Scheme IThe Fmoc protecting group is typically removed with piperidine (Scheme II) , a heterocyclic secondary amine.
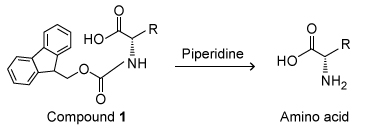 Scheme IIBecause the use of piperidine is restricted, structurally similar amines were examined as possible replacements. To determine whether piperidine analogues were suitable alternatives for Fmoc removal, deprotection of Compound 2 was carried out with several amine bases (Scheme III) .
Scheme IIBecause the use of piperidine is restricted, structurally similar amines were examined as possible replacements. To determine whether piperidine analogues were suitable alternatives for Fmoc removal, deprotection of Compound 2 was carried out with several amine bases (Scheme III) .
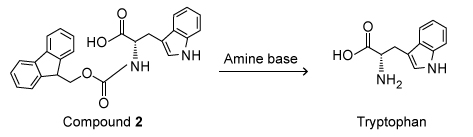 Scheme IIICompound 2 was prepared from L-tryptophan and Fmoc-Cl, and the reaction was monitored by thin-layer chromatography (TLC) and visualized by ultraviolet light. The deprotections were done with piperidine and each analogue using a microwave set to 155 watts at 75 °C for 15 seconds. The products of the deprotections were analyzed by high-performance liquid chromatography (HPLC) and mass spectrometry; the retention times of the products were compared to tryptophan and Compound 2 standards.
Scheme IIICompound 2 was prepared from L-tryptophan and Fmoc-Cl, and the reaction was monitored by thin-layer chromatography (TLC) and visualized by ultraviolet light. The deprotections were done with piperidine and each analogue using a microwave set to 155 watts at 75 °C for 15 seconds. The products of the deprotections were analyzed by high-performance liquid chromatography (HPLC) and mass spectrometry; the retention times of the products were compared to tryptophan and Compound 2 standards.
Adapted from O.P. Luna et al, "Deprotection Reagents in Fmoc Solid Phase Peptide Synthesis: Moving Away from Piperidine?." Molecules. ©2016 MDPI.
-What can be inferred about the HPLC column used in the passage if tryptophan has a shorter retention time than Compound 2?
A) A reverse-phase HPLC column was used with a nonpolar stationary phase and polar mobile phase.
B) A reverse-phase HPLC column was used with a polar stationary phase and nonpolar mobile phase.
C) A normal-phase HPLC column was used with a polar stationary phase and nonpolar mobile phase.
D) A normal-phase HPLC column was used with a nonpolar stationary phase and polar mobile phase.
Correct Answer:
Verified
Q142: Passage
The protozoan Euglena gracilis expresses glycosyltransferases (enzymes
Q143: Passage
Malaria is caused by the parasite Plasmodium
Q144: Passage
The N-terminus of an amino acid must
Q145: Passage
The N-terminus of an amino acid must
Q146: Passage
Malaria is caused by the parasite Plasmodium
Q148: A carboxylic acid is reacted with NaOH
Q149: Passage
Malaria is caused by the parasite Plasmodium
Q150: Passage
The protozoan Euglena gracilis expresses glycosyltransferases (enzymes
Q151: Thin-layer chromatography was used to determine the
Q152: Passage
The N-terminus of an amino acid must
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents