If 16.87 mL of 0.165 M NaOH solution is titrated witH49.70 mL of H₂ SO₄. What is the molar concentration of the H₂ SO₄solution?
2 NaOH (aq) + H₂ SO₄(aq) Na2 SO₄(aq) + 2 H₂ O ( 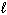 )
)
A) 0.0280 M
B) 0.0560 M
C) 0.112 M
D) 0.243 M
E) 0.972 M
Correct Answer:
Verified
Q97: What is the molarity of a HCl
Q98: In the titration of a 50.0 mL
Q99: What volume of 0.128 M HCl is
Q100: How many mL of 1.0 molar HNO3
Q101: What is the percentage of calcium in
Q102: Exhibit 4-3 Consider the Acid-Base neutralization of
Q103: What is the molarity of a HCl
Q104: What is the percent of chlorine in
Q105: What is the molarity of a NaOH
Q107: What is the molarity of a NaCl
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents