If a sample of methane gas,CH₄,reacts completely with 1.55 L oxygen gas at STP,what mass of methane reacted? CH₄(g) + O₂(g) 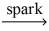 CO₂(g) + H₂O(l)
CO₂(g) + H₂O(l)
A) 0.554 g
B) 1.09 g
C) 1.11 g
D) 2.11 g
E) 2.17 g
Correct Answer:
Verified
Q69: Given that 25.0 mL of 0.100 M
Q70: Given that 50.0 mL of 0.125 M
Q71: Given the three-step "blast furnace" process that
Q72: Given the two-step process for converting aqueous
Q73: Given the three-step Ostwald process for manufacturing
Q75: Given 25.0 mL of 0.200 M H₂SO₄,how
Q76: Given the three-step Solvay process for manufacturing
Q77: Given that 25.0 mL of 0.500 M
Q78: Given that 0.500 g of NaHCO₃ reacts
Q79: Given that 0.500 g of NaHCO₃ reacts
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents